Photo from wikipedia
In the present study, we evaluated the performance of different protocols for the hepatic differentiation of human‐induced pluripotent stem cells (hiPSCs) in microfluidic biochips. Strategies for complete and partial on‐chip… Click to show full abstract
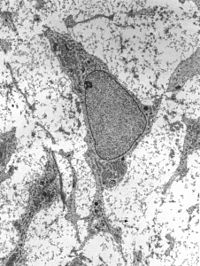
In the present study, we evaluated the performance of different protocols for the hepatic differentiation of human‐induced pluripotent stem cells (hiPSCs) in microfluidic biochips. Strategies for complete and partial on‐chip differentiation were tested. Unlike full on‐chip differentiation, the transfer of iPSCs from Petri dishes to biochips during the differentiation process produced a heterogeneous tissue with enhanced hepatic features compared with control cultures in Petri dishes. The tissue in biochips was constituted of cells expressing either stabilin‐1 or albumin, while no stabilin‐1 was detected in controls. Functional analysis also revealed double the production rate for albumin in biochips (about 2,000 ng per day per 106 cells). Besides this, tissues obtained in biochips and controls exhibited the metabolism of a specific bile acid. Whole transcriptome analysis with nanoCAGE exhibited a differential expression of 302 genes between control and biochip cultures and a higher degree of hepatic differentiation in biochips, together with increased promoter motif activity for typical liver transcription factors such as estrogen related receptor alpha ( ESRRA), hepatic nuclear factor 1 ( HNF1A), hepatic nuclear factor 4 ( HNF4A), transcription factor 4 ( TCF4), and CCAAT enhancer binding protein alpha ( CEBPA). Gene set enrichment analysis identified several pathways related to the extracellular matrix, tissue reorganization, hypoxia‐inducible transcription factor, and glycolysis that were differentially modulated in biochip cultures. However, the presence of CK19/ALB‐positive cells and the ɑ‐fetoprotein levels measured in the cultures still reflect primitive differentiation patterns. Overall, we identified key parameters for improved hepatic differentiation on‐chip, including the maturation stage of hepatic progenitors, inoculation density, adhesion time, and perfusion flow rate. Optimization of these parameters further led to establish a protocol for reproducible differentiation of hiPSCs into hepatocyte‐like cells in microfluidic biochips with significant improvements over Petri dish cultures.
Journal Title: Biotechnology and Bioengineering
Year Published: 2019
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!