Photo from wikipedia
An asymmetric organocatalytic Michael addition–cyclization cascade reaction has been developed using cyclopentane-1,2-dione as a Michael donor and α,β-unsaturated aldehydes as Michael acceptors. Bicyclic hemiacetals were obtained in excellent yields and… Click to show full abstract
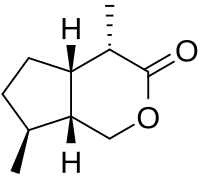
An asymmetric organocatalytic Michael addition–cyclization cascade reaction has been developed using cyclopentane-1,2-dione as a Michael donor and α,β-unsaturated aldehydes as Michael acceptors. Bicyclic hemiacetals were obtained in excellent yields and enantioselectivities. On the basis of the results, a one-pot reaction has been developed to obtain chiral 3-substituted cyclopentane-1,2-diones and substituted dihydropyrans in good yields and excellent enantioselectivity.
Journal Title: Synthesis
Year Published: 2017
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!