Photo from wikipedia
As a SNARE binding protein, tomosyn has been reported to negatively regulate synaptic exocytosis via arresting syntaxin‐1 and SNAP‐25 into a nonfusogenic product that precludes synaptobrevin‐2 entry, raising the question… Click to show full abstract
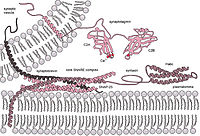
As a SNARE binding protein, tomosyn has been reported to negatively regulate synaptic exocytosis via arresting syntaxin‐1 and SNAP‐25 into a nonfusogenic product that precludes synaptobrevin‐2 entry, raising the question how the assembly of the SNARE complex is achieved. Here, we have investigated new functions of tomosyn in SNARE complex formation and SNARE‐mediated vesicle fusion. Assisted by NSF/α‐SNAP, syntaxin‐1 escapes tomosyn arrest and assembles into the Munc18‐1/syntaxin‐1 complex. Munc13‐1 then catalyzes the transit of syntaxin‐1 from the Munc18‐1/syntaxin‐1 complex to the SNARE complex in a manner specific to synaptobrevin‐2 but resistant to tomosyn. Our data suggest that tomosyn ensures SNARE assembly in a way amenable to tight regulation by Munc18‐1 and Munc13‐1.
Journal Title: FEBS Letters
Year Published: 2018
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!