Photo from wikipedia
Cytoplasmic domains frequently promote functional assembly of multimeric ion channels. To investigate structural determinants of this process, we generated the ‘T1‐chimera’ construct of the NaChBac sodium channel by truncating its… Click to show full abstract
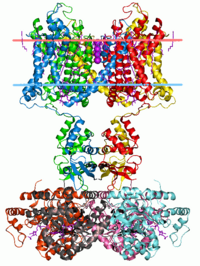
Cytoplasmic domains frequently promote functional assembly of multimeric ion channels. To investigate structural determinants of this process, we generated the ‘T1‐chimera’ construct of the NaChBac sodium channel by truncating its C‐terminal domain and splicing the T1‐tetramerisation domain of the Kv1.2 channel to the N terminus. Purified T1‐chimera channels were tetrameric, conducted Na+ when reconstituted into proteoliposomes, and were functionally blocked by the drug mibefradil. Both the T1‐chimera and full‐length NaChBac had comparable expression levels in the membrane, whereas a NaChBac mutant lacking a cytoplasmic domain had greatly reduced membrane expression. Our findings support a model whereby bringing the transmembrane regions into close proximity enables their tetramerisation. This phenomenon is found with other channels, and thus, our findings substantiate this as a common assembly mechanism.
Journal Title: Febs Letters
Year Published: 2022
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!