Photo from wikipedia
The cAMP‐dependent protein kinase A (PKA) is the archetypical eukaryotic kinase. The catalytic subunit (PKA‐C) structure is highly conserved among the AGC‐kinase family. PKA‐C is a bilobal enzyme with a… Click to show full abstract
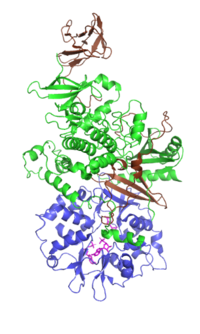
The cAMP‐dependent protein kinase A (PKA) is the archetypical eukaryotic kinase. The catalytic subunit (PKA‐C) structure is highly conserved among the AGC‐kinase family. PKA‐C is a bilobal enzyme with a dynamic N‐lobe, harbouring the Adenosine‐5′‐triphosphate (ATP) binding site and a more rigid helical C‐lobe. The substrate‐binding groove resides at the interface of the two lobes. A distinct feature of PKA‐C is the positive binding cooperativity between nucleotide and substrate. Several PKA‐C mutations lead to the development of adenocarcinomas, myxomas, and other rare forms of liver tumours. Nuclear magnetic resonance (NMR) spectroscopy shows that these mutations disrupt the allosteric communication between the two lobes, causing a drastic decrease in binding cooperativity. The loss of cooperativity correlates with changes in substrate fidelity and reduced kinase affinity for the endogenous protein kinase inhibitor (PKI). The similarity between PKI and the inhibitory sequence of the kinase regulatory subunits suggests that the overall mechanism of regulation of the kinase may be disrupted. We surmise that a reduced or obliterated cooperativity may constitute a common trait for both orthosteric and allosteric mutations of PKA‐C that may lead to dysregulation and disease.
Journal Title: FEBS Letters
Year Published: 2023
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!