Photo from wikipedia
Insulin secretion is a signal‐triggered process that requires membrane fusion between the secretory granules and plasma membrane in pancreatic β cells. The exocytosis of insulin is mediated by target‐soluble N‐ethylmaleimide… Click to show full abstract
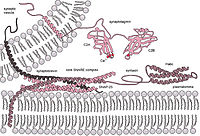
Insulin secretion is a signal‐triggered process that requires membrane fusion between the secretory granules and plasma membrane in pancreatic β cells. The exocytosis of insulin is mediated by target‐soluble N‐ethylmaleimide sensitive factor attachment protein receptors (SNAREs) on the plasma membrane and vesicle‐SNAREs on the vesicles, which assemble into a quaternary trans‐SNARE complex to initiate the fusion. Expression of fusion proteins is reduced in the islets of patients with type II diabetes, indicating that SNARE‐mediated fusion defect is closely related to insulin‐based metabolic diseases. Previous studies have suggested that epigallocatechin gallate (EGCG) has an inhibitory effect on membrane fusion. In the present study, we performed in vitro reconstitution assays to unravel the molecular mechanisms of EGCG in SNARE‐mediated insulin secretory vesicle fusion. Our data show that EGCG efficiently inhibits insulin secretory SNARE‐mediated membrane fusion. Mechanistic studies indicated that EGCG blocks the formation of the trans‐SNARE complex. Furthermore, calcium/synaptotagmin‐7‐stimulated fusion kinetics were largely reduced by EGCG, confirming that it is a potential regulator of SNARE‐dependent insulin secretion. Our findings suggest that the trans‐SNARE complex might be a promising target for controlling SNARE‐dependent vesicle fusion.
Journal Title: FEBS Open Bio
Year Published: 2022
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!