Photo from wikipedia
Heme‐nitric oxide/oxygen binding (H‐NOX) proteins are a family of gas‐binding hemoproteins that bind diatomic gas ligands such as nitric oxide (NO) and oxygen (O2). In bacteria, H‐NOXs are often associated… Click to show full abstract
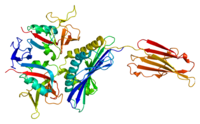
Heme‐nitric oxide/oxygen binding (H‐NOX) proteins are a family of gas‐binding hemoproteins that bind diatomic gas ligands such as nitric oxide (NO) and oxygen (O2). In bacteria, H‐NOXs are often associated with signaling partners, including histidine kinases (HKs), diguanylate cyclases (DGCs) or methyl‐accepting chemotaxis proteins (MCPs), either as a stand‐alone protein or as a domain of a larger polypeptide. H‐NOXs regulate the activity of cognate signaling proteins through ligand‐induced conformational changes in the H‐NOX domain and protein/protein interactions between the H‐NOX and the cognate signaling partner. This review summarizes recent progress toward deciphering the molecular mechanism of bacterial H‐NOX activation and the subsequent regulation of H‐NOX‐associated cognate sensor proteins from a structural and biochemical point of view.
Journal Title: ChemBioChem
Year Published: 2019
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!