Photo from wikipedia
Endovascular therapy offers a less invasive treatment option compared to surgery, and therefore, has become the therapy of choice in many patients with peripheral artery disease (PAD). Restenosis due to… Click to show full abstract
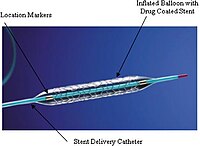
Endovascular therapy offers a less invasive treatment option compared to surgery, and therefore, has become the therapy of choice in many patients with peripheral artery disease (PAD). Restenosis due to neointimal hyperplasia; however, is a challenge, despite the high initial procedural success rate. Consequently, drug-coated balloons (DCBs) and drug-eluting stents (DESs), which allows local application of antiproliferative molecules, such as paclitaxel has been designed to tackle this important issue. Based on the higher patency rates shown in multiple clinical trials, paclitaxel-coated devices have been widely adopted by many operators for the treatment of femoropopliteal disease. However, in December 2018, a meta-analysis published by Katsanos and colleagues raised red flags about the safety of these devices. The meta-analysis included more than 4,600 patients from 28 randomized controlled trials (RCTs) using different paclitaxel-coated balloon and stents in femoropopliteal lesions. While the authors found similar allcause death in the paclitaxel and the control groups at 1 year, there was a significant increase in mortality beyond this point. All-cause death was significantly higher in paclitaxel group at 2 years (7.2 vs. 3.8%) and increased even further in the paclitaxel group (14.7%) compared to the controls (8.1%) at 5 years with a number-needed-toharm was as low as 14. The study was criticized for its shortcomings, such as very short peer review time, direct acceptance of the paper without any revisions, including but not limited to use of different device platforms, the lack of patient-level data, the lack of a plausible causal explanation, substantial loss to follow-up, and intention-totreat data analysis along with significant number of cross overs between the groups. However, understandably the results created a subsequent controversy within the field and led to a substantial increase in the number of publications on this very important topic. In this issue of the journal, Böhme et al reported their singlecenter mortality rates in patients with symptomatic PAD who received paclitaxel-eluting stents versus controls. Authors retrospectively analyzed 303 patients with intermittent claudication or ischemic rest pain, who were treated with an uncoated device and 296 patients with a DES. Over a mean follow-up period was 51.8 ± 23.4 months, mortality incidence was significantly higher after uncoated treatment (32.3%) than after DES (22.6%). Mortality incidence was similar between the groups, when the groups were matched by propensity scores (32.5 vs. 24.1%). Of note, these mortality rates were remarkably higher than those in the meta-analysis (14.7 and 8.1% at 5 years), which was attributed to the exclusion of higher-risk patients from the RCTs, raising further concerns on the applicability of the results of the meta-analysis to the real-world. The same research group also published their real-world data on paclitaxel-coated balloons recently and demonstrated a long-term mortality benefit after DCB angioplasty compared to uncoated balloon angioplasty of femoropopliteal lesions. Authors also explored the relationship between paclitaxel dose and the mortality risk. The patients treated with Zilver PTX were divided into 3 groups according to the paclitaxel dosage (<1,500 μg, 1,500–2,500 μg and >2,500 μg) and found no mortality difference between the groups. These results also contradict with the results of Katsanos et al, which showed a significant relationship between exposure to paclitaxel (dose-time product) and absolute risk of death, despite concerns on their paclitaxel dose calculation methods. Moreover, when follow-up interventions were taken into account to calculate a total paclitaxel body exposure, interestingly Böhme and colleagues showed a significantly lower mortality for the higher Received: 26 October 2020 Accepted: 27 October 2020
Journal Title: Catheterization and Cardiovascular Interventions
Year Published: 2020
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!