Photo from wikipedia
Zinc chalcogenides nanostructures are promising newly‐studied materials for photocatalytic reactions such as water reduction for hydrogen generation and photoinitiation of radical polymerization processes. Herein, we introduced a straightforward synthesis of… Click to show full abstract
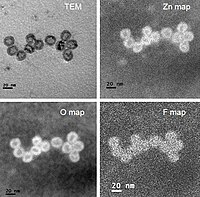
Zinc chalcogenides nanostructures are promising newly‐studied materials for photocatalytic reactions such as water reduction for hydrogen generation and photoinitiation of radical polymerization processes. Herein, we introduced a straightforward synthesis of thin ZnS shell on ZnSe nanorods and characterized the resulting core/shell ZnSe/ZnS nanorods by electron microscopy techniques and X‐ray photoelectron spectroscopy. Then, the photocatalytic activities of the nanorods were studied by gas chromatography for hydrogen generation and by Fourier transform infrared spectroscopy for the photoinitiation capability. While such core/shell systems may limit charge transfer to the solution due to the type‐I band alignment, we find that the photochemical stability and photocatalytic activity of this system is significantly enhanced over pristine ZnSe nanorods. ZnSe/ZnS nanorods with Ni(NO3)2 co‐catalyst are used for hydrogen generation and the effects of the nanorods’ surface coating, pH conditions and type of hole scavenger are examined. The best performances were observed for nanorods after ligand stripping with Meerwein′s reagent using ascorbic acid as a hole acceptor at pH 4.5. All three parameters were found to affect the photocatalytic activity. Yet, the surface coating and pH dependence differ from previous reports on cadmium chalcogenides photocatalysts. The origin of their differences is discussed and attributed to the band alignment of the systems and the nature of the co‐catalyst. The stable heavy‐metal free ZnSe/ZnS nanorods system presented here holds promise for various photocatalytic applications.
Journal Title: ChemCatChem
Year Published: 2019
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!