Photo from wikipedia
The tropical diseases human African trypanosomiasis, Chagas disease, and the various forms of leishmaniasis are caused by parasites of the family of trypanosomatids. These protozoa possess a unique redox metabolism… Click to show full abstract
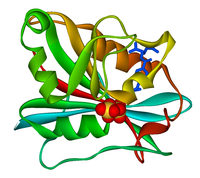
The tropical diseases human African trypanosomiasis, Chagas disease, and the various forms of leishmaniasis are caused by parasites of the family of trypanosomatids. These protozoa possess a unique redox metabolism based on trypanothione and trypanothione reductase (TR), making TR a promising drug target. We report the optimization of properties and potency of cyclohexylpyrrolidine inhibitors of TR by structure‐based design. The best inhibitors were freely soluble and showed competitive inhibition constants (Ki) against Trypanosoma (T.) brucei TR and T. cruzi TR and in vitro activities (half‐maximal inhibitory concentration, IC50) against these parasites in the low micromolar range, with high selectivity against human glutathione reductase. X‐ray co‐crystal structures confirmed the binding of the ligands to the hydrophobic wall of the “mepacrine binding site” with the new, solubility‐providing vectors oriented toward the surface of the large active site.
Journal Title: ChemMedChem
Year Published: 2018
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!