Photo from wikipedia
Knoevenagel condensation is an entrenched, prevailing, prominent arsenal following greener principles in the generation of α, β‐unsaturated ketones/carboxylic acids by involving carbonyl functionalities and active methylenes. This reaction has proved… Click to show full abstract
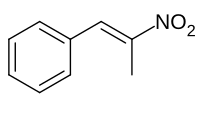
Knoevenagel condensation is an entrenched, prevailing, prominent arsenal following greener principles in the generation of α, β‐unsaturated ketones/carboxylic acids by involving carbonyl functionalities and active methylenes. This reaction has proved to be a major driving force in many multicomponent reactions indicating the prolific utility toward the development of biologically fascinating molecules. This eminent reaction was acclimatised on different pharmacophoric aldehydes (benzimidazole, β‐carboline, phenanthrene, indole, imidazothiadiazole, pyrazole etc.) and active methylenes (oxindole, barbituric acid, Meldrum's acid, thiazolidinedione etc.) to generate the library of chemical compounds. Their potential was also explicit to understand the significance of functionalities involved, which thereby evoke further developments in drug discovery. Furthermore, most of these reaction products exhibited remarkable anticancer activity in nanomolar to micromolar ranges by targeting different cancer targets like DNA, microtubules, Topo‐I/II, and kinases (PIM, PARP, NMP, p300/CBP) etc. This review underscores the efficiency of the Knoevenagel condensation explored in the past six‐year to generate molecules of pharmacological interest, predominantly toward cancer. The present review also provides the aspects of structure‐activity relationships, mode of action and docking study with possible interaction with the target protein.
Journal Title: ChemMedChem
Year Published: 2022
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!