Photo from wikipedia
The development of green solvents is one of the key tenets of Green Chemistry as solvents account for the majority of waste stemming from the production of the chemicals on… Click to show full abstract
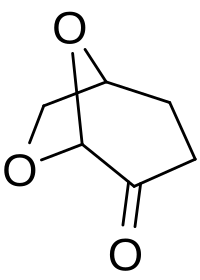
The development of green solvents is one of the key tenets of Green Chemistry as solvents account for the majority of waste stemming from the production of the chemicals on which we have all come to rely. An important class of solvents is the dipolar aprotics, which include N,N-dimethylformamide (DMF) and N-methyl-2-pyrrolidone (NMP). In addition to being derived from non-renewable resources, these solvents are also under increased regulatory pressures that will limit their industrial applications. This Concept concerns the bio-available solvent Cyrene (dihydrolevoglucosenone) as a potential replacement for toxic dipolar aprotic solvents. An emphasis is placed on examining the strengths and weaknesses of Cyrene as a solvent and is accomplished by looking at the synthesis, derivatization, and application in synthetic protocols of Cyrene. With respect to the Twelve Principles of Green Chemistry, this Concept describes a bio-available solvent that should have a disruptive effect on the use of traditional industrial dipolar aprotic solvents.
Journal Title: ChemSusChem
Year Published: 2018
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!