Photo from wikipedia
Cardiac glycosides digoxin and digitoxin are used in therapy for the treatment of congestive heart failure. Moreover, these compounds can be responsible for intoxication cases caused by fortuitous ingestion of… Click to show full abstract
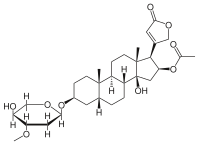
Cardiac glycosides digoxin and digitoxin are used in therapy for the treatment of congestive heart failure. Moreover, these compounds can be responsible for intoxication cases caused by fortuitous ingestion of leaves of Digitalis. Due to the narrow therapeutic range of these drugs, therapeutic drug monitoring is recommended in the clinical practice. In this context, immunoassays‐based methods are generally employed but digoxin‐ and digitoxin‐like compounds can interfere with the analysis. The aim of this study was to develop and validate an original UPLC–MS/MS method for the determination of digoxin and digitoxin in plasma. The method shows adequate sensitivity and selectivity with acceptable matrix effects and very good linearity, accuracy, precision, and recovery. A simple liquid–liquid extraction procedure was used for sample clean‐up. The method was applied for the analysis of n = 220 plasma samples collected in two different clinical chemistry laboratories and previously tested by the same immunoassay. The statistical comparison showed a relevant negative bias of the UPLC–MS/MS method versus the immunoassay. These results are consistent with an immunoassay overestimation of digoxin plasmatic levels due to cross‐reaction events with endogenous digoxin‐like substances.
Journal Title: Electrophoresis
Year Published: 2022
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!