Photo from wikipedia
The asthmatic airways are highly susceptible to inflammatory injury by air pollutants such as ozone (O3), characterized by enhanced activation of eosinophilic granulocytes and a failure of immune protective mechanisms.… Click to show full abstract
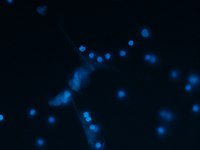
The asthmatic airways are highly susceptible to inflammatory injury by air pollutants such as ozone (O3), characterized by enhanced activation of eosinophilic granulocytes and a failure of immune protective mechanisms. Eosinophil activation during asthma exacerbation contributes to the proinflammatory oxidative stress by high levels of nitric oxide (NO) production and extracellular DNA release. Surfactant protein‐D (SP‐D), an epithelial cell product of the airways, is a critical immune regulatory molecule with a multimeric structure susceptible to oxidative modifications. Using recombinant proteins and confocal imaging, we demonstrate here that SP‐D directly bound to the membrane and inhibited extracellular DNA trap formation by human and murine eosinophils in a concentration and carbohydrate‐dependent manner. Combined allergic airway sensitization and O3 exposure heightened eosinophilia and nos2 mRNA (iNOS) activation in the lung tissue and S‐nitrosylation related de‐oligomerisation of SP‐D in the airways. In vitro reproduction of the iNOS action led to similar effects on SP‐D. Importantly, S‐nitrosylation abolished the ability of SP‐D to block extracellular DNA trap formation. Thus, the homeostatic negative regulatory feedback between SP‐D and eosinophils is destroyed by the NO‐rich oxidative lung tissue environment in asthma exacerbations.
Journal Title: Journal of Leukocyte Biology
Year Published: 2018
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!