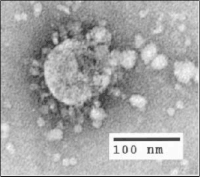
Photo from wikipedia
The first reported case of Middle East respiratory syndrome coronavirus (MERS‐CoV) infection was identified in Saudi Arabia in September 2012, since which time there have been over 2000 laboratory‐confirmed cases,… Click to show full abstract
The first reported case of Middle East respiratory syndrome coronavirus (MERS‐CoV) infection was identified in Saudi Arabia in September 2012, since which time there have been over 2000 laboratory‐confirmed cases, including 750 deaths in 27 countries. Nucleic acid testing (NAT) is the preferred method for the detection of MERS‐CoV. A single round of a Proficiency Testing Program (PTP) was used to assess the capability of laboratories globally to accurately detect the presence of MERS‐CoV using NAT. A panel of eleven lyophilized specimens containing different viral loads of MERS‐CoV, common coronaviruses, and in vitro RNA transcripts was distributed to laboratories in all six World Health Organization regions. A total of 96 laboratories from 79 countries participating in the PTP, with 76 of 96 (79.2%) reporting correct MERS‐CoV results for all nine scored specimens. A further 10 laboratories (10.4%) scored correctly in eight of nine specimens of the PTP. The majority of laboratories demonstrated satisfactory performance in detecting the presence of MERS‐CoV using NAT. However, some laboratories require improved assay sensitivity, reduced cross contamination of samples, and improved speciation of coronavirus subtypes for potentially complex clinical specimens. Further PTP and enhanced links with expert laboratories globally may improve the laboratory performance.
Journal Title: Journal of Medical Virology
Year Published: 2018
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!