Photo from wikipedia
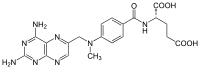
Methotrexate (MTX) is an essential drug in the treatment of acute lymphoblastic leukemia (ALL). High-dose methotrexate (HD-MTX) administration (> 1 g/m) can lead to its tubular precipitation, leading to acute… Click to show full abstract
Methotrexate (MTX) is an essential drug in the treatment of acute lymphoblastic leukemia (ALL). High-dose methotrexate (HD-MTX) administration (> 1 g/m) can lead to its tubular precipitation, leading to acute kidney injury (AKI) and other toxicities [1]. Methotrexate plasma concentrations are routinely checked after HD-MTX infusion. There are well-documented data on association between MTX toxicity and impaired function of several transporters from the ATP-binding cassette (ABC) and the solute carrier (SLC) transporter superfamilies [1–6]. Here, we present a 26-year-old male with B cell ALL whose clinical course after the first HD-MTX infusion (5000 mg/m IV) was uneventful. However, after the second infusion 14 days later, he suffered damage to four organ systems, probably due to combination of genetic predisposition and pharmacokinetic interactions between MTX and concomitant drugs. Forty-eight hours after the second HD-MTX infusion, extremely high MTX serum concentration (142.56 μmol/L) was observed and slow MTX elimination ensued during the next 16 days. During this hospital stay, the patient was also treated with pantoprazole, ketoprofen, ciprofloxacin, and later with piperacillin/tazobactam, while he received none of these during the first hospitalization. Subsequently, the patient developed AKI (with a fall of estimated glomerular filtration rate to 45 mL/min/1.73 m), severe neutropenia, and a rise in alanine aminotransferase (234 U/L). With support ive care (ant ibiot ics , hyperhydrat ion, ur ine alkalization, leucovorin rescue, filgrastim), MTX concentration gradually decreased, renal function partially recovered and neutropenia resolved, and the patient was discharged in good clinical condition. Two weeks later, he presented to our Emergency Unit with confusion, headache, nausea, vomiting, and multiple generalized tonicclonic seizures. The patient was hypertensive (185/ 120 mmHg), eupneic, and in sinus rhythm (80/min). Magnetic resonance imaging (MRI) showed bilateral, symmetric cortical, and subcortical zones of vasogenic edema in medial parts of temporal lobes (Fig. 1), together with restriction of diffusion cortically in the posterior part of the left parietal lobe and, less pronounced, in medial parts of parietal lobes (consistent with cytotoxic edema). These findings and the clinical presentation pointed to the posterior reversible encephalopathy syndrome (PRES). Treatment with antiepileptics resulted in cessation of seizures. Since all of the aforementioned conditions (AKI, agranulocytosis, drug-induced liver injury, and PRES) were probably caused by extremely high plasma MTX concentration and prolonged elimination, it was decided to perform pharmacogenetic analyses. It was found that the patient is a carrier of variant—low activity alleles of several drug transporters, known to be important for MTX distribution, including transport across the blood-brain barrier (BBB) and renal elimination. The patient was found to be homozygous for SLCO1B1 T521C, ABCB1 G2677T/A, C3435T, C1236T, ABCC2 G1249A, and heterozygous for MTHFR C677T and A1298C. While all of these genetic polymorphisms can predispose to MTX toxicity, probably the most established is the association with C677T polymorphism [7, 8]. A strong genetic predisposition to prolonged MTX elimination in combination with clinically significant pharmacokinetic interactions with * Ela Ćurčić [email protected]
Journal Title: European Journal of Clinical Pharmacology
Year Published: 2018
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!