Photo from wikipedia
Amphiphilic globular protein–polymer hybrids, consisting of poly(methyl methacrylate) (PMMA) as hydrophobic tail and globular protein as hydrophilic head, are prepared by the end-functional PMMA covalently binding to the primary amino… Click to show full abstract
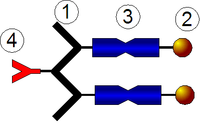
Amphiphilic globular protein–polymer hybrids, consisting of poly(methyl methacrylate) (PMMA) as hydrophobic tail and globular protein as hydrophilic head, are prepared by the end-functional PMMA covalently binding to the primary amino groups of bovine serum albumin (BSA) in the phosphate buffer saline (PBS)/acetone mixture solvents at pH 5. The self-assembly behaviors of amphiphilic BSA–PMMA hybrids are explored in aqueous solution by the dynamic light scatter (DLS), scanning electron microscopy (SEM) and transmission electron microscopy (TEM). The self-assembled morphologies of the amphiphilic BSA–PMMA hybrids are controlled by the length of hydrophobic PMMA chains. The amphiphilic BSA–PMMA hybrids with the longer hydrophobic PMMA chains self-assembled into spherical vesicles and elongated tubular vesicles, and conversely, they self-assembled into micelles. A vesicle consists of BSA as two outer hydrophilic layers and PMMA as hydrophobic wall.Graphical AbstractAmphiphilic globular protein–polymer hybrids consist of PMMA as hydrophobic tail and globular protein BSA as hydrophilic head. Amphiphilic globular protein–polymer hybrids self-assembled into micelles or vesicles in phosphate buffer saline. The self-assembled morphology of amphiphilic protein–polymer hybrids was controlled by adjusting the length of hydrophobic chains.
Journal Title: Polymer Bulletin
Year Published: 2017
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!