Photo from wikipedia
IntroductionThere are no comparative studies available for hyperosmolar therapy in children. The present study is a prospective open label randomized control trial to compare the effect of equiosmolar doses of… Click to show full abstract
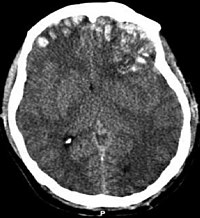
IntroductionThere are no comparative studies available for hyperosmolar therapy in children. The present study is a prospective open label randomized control trial to compare the effect of equiosmolar doses of mannitol and hypertonic saline in reducing intracranial pressure in children who sustained severe traumatic brain injury.MethodsThis is a prospective open-label randomized controlled trial. Thirty children aged less than or equal to 16 years with severe traumatic brain injury and raised intracranial pressure as measured by ventricular catheter insertion were enrolled. Sixteen children received 20% mannitol, and 14 children received 3% saline as 2.5 ml/kg bolus for episodes of intracranial pressure above cutoff value for age. The mean reduction in intracranial pressure and Glasgow outcome scale at 6 months after injury was measured.ResultsThe mean reduction in intracranial pressure in mannitol group was 7.13 mmHg and in hypertonic saline group was 5.67 mmHg, and the difference was not statistically significant, p = 0.33. The incidence of death or survival in vegetative state was 23.07% in mannitol group and 16.66% in hypertonic saline group, and the difference was not statistically significant, p = 0.69.ConclusionBoth mannitol and hypertonic saline were equally effective for treatment of raised intracranial pressure in children with severe traumatic brain injury.
Journal Title: Child's Nervous System
Year Published: 2019
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!