Photo from wikipedia
Quantum chemical calculations were carried out for deprotonated ( P − ) and protonated purine ( PH + ) and for adducts with one alkali metal cation ( P −… Click to show full abstract
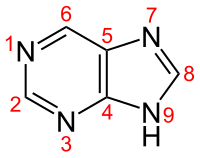
Quantum chemical calculations were carried out for deprotonated ( P − ) and protonated purine ( PH + ) and for adducts with one alkali metal cation ( P − M + and PM + , where M + is Li + or Na + ) in the gas phase {B3LYP/6-311+G(d,p)}, a model of perfectly apolar environment, and for selected structures in aqueous solution {PCM(water)//B3LYP/6-311+G(d,p)}, a reference polar medium for biological studies. All potential isomers of purine derivatives were considered, the favored structures indicated, and the preferred sites for protonation/deprotonation and cationization reactions determined. Proton and metal cation basicities of purine in the gas phase were discussed and compared with those of imidazole and pyrimidine. Bond-length alternations in the P , PH + , P − M + , and PM + forms were quantitatively measured using the harmonic oscillator model of electron delocalization (HOMED) indices and compared with those for P . Variations of the HOMED values when proceeding from the purine structural building blocks, pyrimidine and imidazole, to the bicyclic purine system were also examined. Generally, the isolated NH isomers exhibit a strongly delocalized π-system (HOMED > 0.8). Deprotonation slightly increases the HOMED values, whereas protonation and cationization change the HOMED indices in different way. For bidentate M + -adducts, the HOMED values are larger than 0.9 like for the largely delocalized P − . The HOMED values correlate well in a comprehensive relationship with the relative Gibbs energies (Δ G ) calculated for individual isomers whatever the purine form is, neutral, protonated, or cationized. When PCM-DFT model was utilized for P − , PH + , PM + , and P − M + (M + = Li + ) both electron delocalization and relative stability are different from those for the molecules in vacuo. The solvation effects cause a slight increase in HOMEDs, whereas the Δ E s decrease, but in different ways. Hence, contribution of particular isomers in the isomeric mixtures of PH + , PM + , and P − M + also varies. HOMED variations for the favored neutral, deprotonated, protonated, and lithiated forms of purine in the gas phase and aqueous solution
Journal Title: Journal of Molecular Modeling
Year Published: 2020
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!