Photo from wikipedia
Abstract Generally, the sulfate (SO 4 · − ) and hydroxyl (HO · ) radicals are the dominant active species in most catalytic oxidation processes with peroxymonosulfate (PMS). However, the… Click to show full abstract
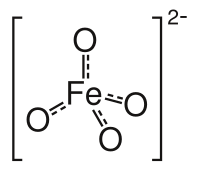
Abstract Generally, the sulfate (SO 4 · − ) and hydroxyl (HO · ) radicals are the dominant active species in most catalytic oxidation processes with peroxymonosulfate (PMS). However, the existence of various natural organic and inorganic matters in aquatic environments might influence the oxidation efficiency of these radicals, and/or form more toxic and refractory intermediates than the parent, especially in chlorine-ion-containing conditions. Here, we constructed a novel visible-light catalytic system with PMS based on iron hexadecachlorophthalocyanine-poly (4-vinylpyridine)/polyacrylonitrile nanofibers through pyridine ligands to generate high-valent iron-oxo (Fe(IV)=O) species as the main active species. The coordination structure was characterized by UV–Vis diffuse reflection, X-ray photoelectron spectroscopy, etc. The high-valent iron-oxo generation from peroxysulfate O–O bond heterolytic cleavage was proved by high-definition electrospray ionization mass spectrometer. Ultra-performance liquid chromatography coupled with high-definition mass spectrometry showed that the photocatalytic system was efficient for the degradation of carbamazepine and the chlorinated intermediates by iron-oxo active species in chlorine-ion-containing conditions. Graphic Abstract
Journal Title: Catalysis Letters
Year Published: 2019
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!