Photo from wikipedia
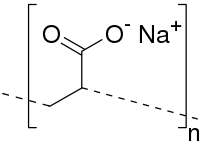
Novel magnetic sodium alginate-based biopolymer Fe3O4@SA-Fe was successfully prepared by the cross-linking reaction of sodium alginate (SA) and Fe(III) ions and adding magnetic ferric oxide (Fe3O4), and characterized by Scanning… Click to show full abstract
Novel magnetic sodium alginate-based biopolymer Fe3O4@SA-Fe was successfully prepared by the cross-linking reaction of sodium alginate (SA) and Fe(III) ions and adding magnetic ferric oxide (Fe3O4), and characterized by Scanning Electron Microscopy (SEM), Energy Dispersive Spectroscopy (EDS), UV-visible diffuse reflectance spectroscopy (UV-Vis DRS), Fourier Transform-Infrared (FTIR), X-ray Photoelectron Spectroscopy (XPS) and Vibrating Sample Magnetometer (VSM), respectively. The effects of preparation and adsorption conditions on the adsorbent were investigated by adsorbing Congo red (CR) and Direct red 23 (DR 23) dyes. The results showed that the synthesized Fe3O4@SA-Fe polymer gel beads exhibited super-high adsorption property and good stability when the mass concentrations of SA, Fe(III) ions and Fe3O4 are 16, 12.5 and 7 g/L at room temperature respectively. The adsorption rates of two dyes by Fe3O4@SA-Fe were very fast at 298 K, and the time required to reach the equilibrium was very short, which was 30 min for CR and 60 min for DR 23. Moreover, the dye removal efficiencies were over 99.4% and 95.2% in a wide pH range of 2.0 ~ 9.0 for CR and 2.0 ~ 10.0 for DR 23, respectively. The adsorption process could be accurately described by the pseudo-second-order rate model, which were mostly controlled by intra-particle diffusion. The fitting results of isothermic data by Langmuir, Freundlich and Dubinin-Radushkevich (D-R) models revealed that the equilibrium data completely obeyed the Langmuir model and the obtained maximum adsorption capacities of CR and DR 23 were 3333 and 1429 mg/g at 298 K, respectively. FTIR, UV-Vis and XPS analysis indicated that the electrostatic adsorption, hydrogen bonding and ligand exchange promoted the interaction between dye molecules and Fe3O4@SA-Fe. A green magnetic biosorbent Fe3O4@SA-Fe gel beads with simple preparation method and high-cost performance can be used for super-efficient purification of high-concentration dye effluent and quickly separated from the aqueous phase and recovered, which would have a good application prospect.
Journal Title: Journal of Polymers and the Environment
Year Published: 2020
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!