Photo from wikipedia
Peripheral artery disease (PAD) is a common, debilitating disease that impacts 8.5 million Americans and carries a poor prognosis. The most common manifestation of lower extremity PAD is claudication—a condition… Click to show full abstract
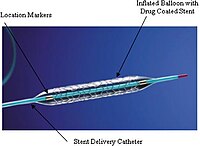
Peripheral artery disease (PAD) is a common, debilitating disease that impacts 8.5 million Americans and carries a poor prognosis. The most common manifestation of lower extremity PAD is claudication—a condition which significantly reduces quality of life and functional status. Paclitaxel-coated balloons and stents (PCBs and PESs) represented a breakthrough in the ability to treat medication-refractory patients relative to bare metal stents (BMSs) and percutaneous transluminal angioplasty (PTA) because they improve primary patency rates, reduce target lesion revascularization (TLR), and minimize late-lumen loss for femoropopliteal lesions. As a result, paclitaxel-coated devices (PCDs) were swiftly established as the standard of care for revascularization of femoropopliteal artery disease. A recent meta-analysis of summary-level data demonstrated a late mortality signal for patients treated with paclitaxel-coated devices relative to uncoated devices. This has had a major impact on the vascular community and for the treatment of patients with PAD. Herein, we provide a detailed review of the available data on the late mortality signal associated with paclitaxel. In December of 2018, Katsanos et al. J Am Heart Assoc 7: e011245, 2018) published data from randomized-controlled trials (RCTs) that demonstrated an increase in mortality at 2 and 5 years in patients treated with PCDs involving the femoropopliteal arterial segment relative to patients treated with uncoated devices. As a result of this analysis, randomized trials were stopped and the FDA sent a letter to healthcare providers recommending restriction of use of these devices to patients at the highest risk of restenosis. As additional data emerged supporting the safety of these devices, the FDA organized an advisory committee meeting to review the available data and to determine a pathway forward. The FDA concluded that there were insufficient data to make a final decision regarding the safety of PCDs. They allowed these devices to remain on the market, but with revised safety labeling and updated their letter to healthcare providers to continue to restrict use to patients at highest risk of reintervention. The FDA also called for additional long-term data, including from RCTs and real-world data. To date, an updated patient-level meta-analysis of clinical trial data, RCTs with longer-term follow-up, and large observational studies have been conducted. While meta-analyses conducted using overlapping clinical trial data have found a persistent increase in mortality for those treated with PCDs, individual industry-sponsored RCTs and large observational studies have consistently failed to detect a corresponding mortality increase. To date, no mechanism linking paclitaxel to mortality has been observed. We are currently at an impasse for drawing definitive conclusions regarding the long-term safety of paclitaxel-coated devices. As we await enrollment in ongoing clinical trials, we must proceed with making reasonable decisions for our patients’ care from the available data, as these devices have important clinical implications for our patients. A critical lesson that can be learned from this controversy is that, for future device trials, committing to long-term follow-up is crucial.
Journal Title: Current Cardiology Reports
Year Published: 2021
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!