Photo from wikipedia
The original version of this article unfortunately contained mistakes. Section BIntroduction^, fourth paragraph should read: In Brazil, RDC Resolution 17/2008 (BRASIL 2008), published by ANVISA, the National Health Surveillance Agency,… Click to show full abstract
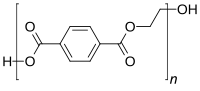
The original version of this article unfortunately contained mistakes. Section BIntroduction^, fourth paragraph should read: In Brazil, RDC Resolution 17/2008 (BRASIL 2008), published by ANVISA, the National Health Surveillance Agency, establishes a positive list of additives for plastic materials intended for the production of packaging and equipment that comes into contact with food and has authorized the use of antimony trioxide in the manufacture of PET resin. It has fixed a specific migration limit for antimony in packaging of 40 μg kg, which is the same limit established by the EU regulation EC 10/2011 (EU 2011), while in Japan, the Japan External Trade Organization has established a limit of 50 μg L (JETRO 2011). The Food and Drug Administration (FDA) has not specified a migration limit for antimony from PET packaging materials (Welle and Franz 2011). The original article was corrected.
Journal Title: Food Analytical Methods
Year Published: 2017
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!