Photo from wikipedia
We are pleased to reply to Lazorwitz et al. [1] letter response in regard to our manuscript published in Advances in Therapy on September 2019 [2]. First, we commend the… Click to show full abstract
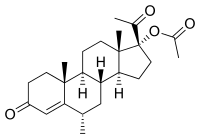
We are pleased to reply to Lazorwitz et al. [1] letter response in regard to our manuscript published in Advances in Therapy on September 2019 [2]. First, we commend the efforts of Lazorwitz A. and colleagues in searching genetic factors that may influence etonogestrel plasma concentrations, especially for exploring Cytochrome P450 (CYP) enzymes as they have traditionally been considered of little relevance for the metabolism of progestogens. The reasons why we decided not to compare our results with theirs were: (a) structural differences between progesterone and etonogestrel (i.e., on position 17, Fig. 1) may alter CYP specificity, (b) the endogenous nature of progesterone vs. the exogenous nature of etonogestrel and (c) the differences in the study population, i.e., postmenopausal women vs. contraceptive implant users. To be able to compare our results with those of Lazorwitz et al. [1], it must be assumed that both compounds are metabolized in the exact same way. More difficult to assume are the limitations stated in ‘‘(b)’’ and ‘‘(c)’’. Postmenopausal women and contraceptive implant users are not comparable in terms of endogenous progesterone plasma levels. In addition, endogenous progesterone may have different effects on exogenous progesterone or etonogestrel metabolism. These differences in the study design and drug characteristics may explain why we found an association between CYP2C19 phenotype and progesterone levels that was not found in their study. Nevertheless, their findings on CYP3A7*1C are surprising. This CYP enzyme has been reported to contribute in a minor extent to progesterone metabolism [3]. However, their findings are coherent, as the presence of this variant leads to the expression of the silenced fetal gene in adults [4]. The latter would increase etonogestrel metabolism, explaining the 23% lower plasma levels reported for CYP3A7*1C carriers compared to wild type patients. It would be of great interest to genotype our study population for this variant. However, due to the low prevalence of this variant and our relatively small sample size, it is highly unlikely that we would observe significant effects. In addition, we did not observe Enhanced Digital Features To view enhanced digital features for this article go to https://doi.org/10.6084/ m9.figshare.11346956.
Journal Title: Advances in Therapy
Year Published: 2019
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!