Photo from wikipedia
The need for sustainability necessitates intensive research on using renewable resources to produce biofuels and biochemicals. The esterification of commercial levulinic acid (LA) into ethyl levulinate (EL) was first optimized… Click to show full abstract
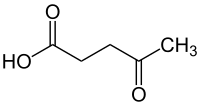
The need for sustainability necessitates intensive research on using renewable resources to produce biofuels and biochemicals. The esterification of commercial levulinic acid (LA) into ethyl levulinate (EL) was first optimized using methanesulfonic acid (MsOH) and response surface methodology (RSM). The optimum condition for the esterification of commercial LA into EL was 5.25 h, 90 °C and 2.75 g of MsOH loading. The EL yield and selectivity obtained were 92.2% and 94%, respectively, at a LA conversion of 98%. The effect of various catalysts which are less corrosive and environmentally friendly, namely MsOH and ionic liquids (ILs), was investigated under fixed conditions to determine the best catalyst for the production of EL from LA. The alkyl levulinate ester selectivity from LA conversion was also studied by using linear and branched chain alcohols. The alkyl levulinate ester, ethyl levulinate, was produced from depithed sugarcane bagasse (DSB)-derived LA using a less corrosive homogenous catalyst (methanesulfonic acid). The reaction conditions of 5.25 h, 90 °C and 2.75 g of MsOH loading were used to produce EL from DSB-derived LA with an EL yield of 75%.
Journal Title: Biomass Conversion and Biorefinery
Year Published: 2021
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!