Photo from wikipedia
L-ascorbic acid, α-tocopherol, procyanidin B3, β-carotene, and astaxanthin are five classic dietary antioxidants. In this study, the interaction between the five antioxidants and human hemoglobin (HHb) was investigated by fluorescence… Click to show full abstract
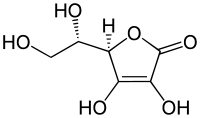
L-ascorbic acid, α-tocopherol, procyanidin B3, β-carotene, and astaxanthin are five classic dietary antioxidants. In this study, the interaction between the five antioxidants and human hemoglobin (HHb) was investigated by fluorescence spectroscopy and molecular modeling. The quenching mechanisms of HHb by the five antioxidants are all static quenching. The downward curvature of the Stern–Volmer plots for HHb–procyanidin B3 system at higher concentrations of procyanidin B3 come from the reason for the variation in the number of accessible tryptophan (Trp) residues toward HHb. The upward curvature of the Stern–Volmer plots for HHb–β-carotene system at higher concentrations of β-carotene predominantly by the “sphere of action” quenching mechanism. The binding constants of HHb with the five antioxidants are in the following order as: astaxanthin > L-ascorbic acid > β-carotene > α-tocopherol > procyanidin B3 at 298 K. The binding processes of the five antioxidants to HHb are all entropy process. Thermodynamic analysis and molecular modeling suggest that the hydrophobic forces are the main interaction force in the binding of the five antioxidants to HHb and hydrogen bond interactions between HHb and L-ascorbic acid/α-tocopherol/procyanidin B3/astaxanthin should be also considered. The fluorescence experimental results are in agreement with the results obtained by molecular modeling study.
Journal Title: Journal of the Iranian Chemical Society
Year Published: 2017
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!