Photo from wikipedia
The equilibrium hetero-association of NADH oxidase and peroxiredoxin was characterized by means of independently conducted measurements of composition-gradient sedimentation equilibrium and composition-gradient static light scattering. Results obtained from both experiments… Click to show full abstract
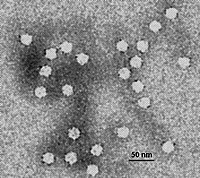
The equilibrium hetero-association of NADH oxidase and peroxiredoxin was characterized by means of independently conducted measurements of composition-gradient sedimentation equilibrium and composition-gradient static light scattering. Results obtained from both experiments were quantitatively accounted for by a model according to which a dimer of NADH oxidase forms a 1:1 equilibrium complex with a decamer of peroxiredoxin under the conditions of these experiments. The best-fit equilibrium constants for heteroassociation of the two proteins obtained from the two measurements were found to be identical to well within the uncertainty of estimate of each of the two methods. The relative virtues of each of the methods are discussed.
Journal Title: Analytical biochemistry
Year Published: 2019
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!