Photo from wikipedia
BACKGROUND AND AIMS The mechanisms that drive atherosclerotic plaque progression and destabilization in humans remain largely unknown. Laboratory models are needed to study these mechanisms under controlled conditions. The aim… Click to show full abstract
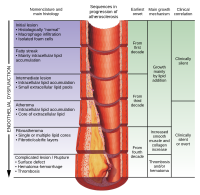
BACKGROUND AND AIMS The mechanisms that drive atherosclerotic plaque progression and destabilization in humans remain largely unknown. Laboratory models are needed to study these mechanisms under controlled conditions. The aim of this study was to establish a new ex vivo model of human atherosclerotic plaques that preserves the main cell types in plaques and the extracellular components in the context of native cytoarchitecture. METHODS Atherosclerotic plaques from carotid arteries of 28 patients undergoing carotid endarterectomy were dissected and cultured. At various time-points, samples were collected and analysed histologically. After enzymatic digestion, single cells were analysed with flow cytometry. Moreover, tissue cytokine production was evaluated. RESULTS We optimised the plaque dissection protocol by cutting plaques into circular segments that we cultured on collagen rafts at the medium-air interface, thus keeping them well oxygenated. With this technique, the relative presence of T and B lymphocytes did not change significantly during culture, and the sizes of lymphocyte subsets remained stable after day 4 of culture. Macrophages, smooth muscle cells, and fibroblasts with collagen fibres, as well as T and B lymphocyte subsets and CD16 natural killer cells, remained largely preserved for 19 days of culture, with a continuous production of inflammatory cytokines and chemokines. CONCLUSIONS Our new model of ex vivo human atherosclerotic plaques, which preserves the main subsets of immune cells in the context of tissue cytoarchitecture, may be used to investigate important aspects of atherogenesis, in particular, the functions of immune cells under controlled laboratory conditions.
Journal Title: Atherosclerosis
Year Published: 2017
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!