Photo from wikipedia
Single vesicle-vesicle fusion assays based on FRET of lipid or content mixing [1-3] are widely used to investigate complexin on vesicle docking [4] and fast fusion process [5]. Most recently,… Click to show full abstract
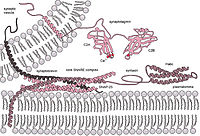
Single vesicle-vesicle fusion assays based on FRET of lipid or content mixing [1-3] are widely used to investigate complexin on vesicle docking [4] and fast fusion process [5]. Most recently, we discovered the important role of its N-terminal domain in promoting fast fusion via membrane interaction [6] and the association of its C-terminus to synaptic vesicles for suppressing spontaneous fusion [7]. For the membrane curvature sensing mechanism of complexin, through molecular dynamics simulations, we found that a high density of membrane defects on synaptic vesicles is responsible for recruiting amphiphilic regions in C-terminal domain of complexin.1. Diao, J. et al. Nat. Protoc. 7, 921 (2012).2. Diao, J. et al. Bioessays 35, 658 (2013).3. Diao, J. et al. Langmuir 25, 7177 (2009).4. Diao, J. et al. J. Am. Chem. Soc. 135, 15274 (2013).5. Lai, Y. et al. Elife 3, e03756 (2014).6. Lai, Y. et al. Proc. Natl. Acad. Sci. USA, 113, E4698 (2016).7. Gong, J., Lai, Y., ⋯ Yang, X., Brunger, A.T., Diao, J. The C-terminal domain of mammalian complexin-1 localizes to highly curved membranes. Proc. Natl. Acad. Sci. USA 113, (2016).
Journal Title: Biophysical Journal
Year Published: 2017
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!