Photo from wikipedia
Fusion of vesicular and plasma membranes is mediated by SNARE proteins. In a vesicular fusion event, the t- and v-SNAREs assemble into a four-helix bundle pulling the two membranes together… Click to show full abstract
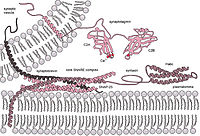
Fusion of vesicular and plasma membranes is mediated by SNARE proteins. In a vesicular fusion event, the t- and v-SNAREs assemble into a four-helix bundle pulling the two membranes together to cause fusion. A decrease in neurotransmitter release upon overexpression of the neuronal protein α-Synuclein (αS) has been observed in animal models, suggesting that αS may act as a regulator of neurotransmission, altering SNARE driven fusion of synaptic vesicles. Recent work in our lab has shown that αS is able to inhibit SNARE-mediated fusion in vitro, although the mechanism appears to be through binding to the lipid bilayer, not through direct interactions with SNARE proteins. Here, we investigate the possibility that αS may also modulate fusion through interactions with SNARE regulatory proteins, synaptotagmin and complexin, using an in vitro fusion assay. We find that in the presence of synaptotagmin and complexin, αS differentially alters SNARE-mediated vesicle fusion in a concentration dependent manner. At low αS concentrations, fusion is significantly enhanced in a concentration-dependent manner (i.e. increasing fusion with increasing αS). However, once a threshold concentration is exceeded, fusion is again inhibited, again in a concentration dependent manner (i.e. decreasing fusion with increasing αS). Direct evidence of protein-protein interactions was monitored using fluorescence correlation spectroscopy to measure the diffusion times of the protein components. SNARE complex formation can be observed as a function of time through an increase in the diffusion time of labeled t-SNARE protein. While αS does not appear to impact the rate of complex formation alone, substantial increases in the diffusion time of the SNARE complex in the presence of αS and complexin were observed, suggesting an interaction between the two. Taken together, our results suggest that αS may have a dual role in SNARE-mediated membrane fusion, as a chaperone of SNARE regulatory components as well as, at high enough concentrations, a fusion inhibitor.
Journal Title: Biophysical Journal
Year Published: 2017
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!