Photo from wikipedia
Abstract Insights into the cobalt sulfide formation upon sulfidation (10 % H2S/H2) of cobalt-based materials with 4 wt % CoO supported on alumina or silica were obtained by Quick-X-ray absorption… Click to show full abstract
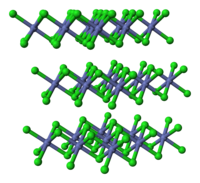
Abstract Insights into the cobalt sulfide formation upon sulfidation (10 % H2S/H2) of cobalt-based materials with 4 wt % CoO supported on alumina or silica were obtained by Quick-X-ray absorption spectroscopy monitoring analysed by multivariate curve regression analysis. A common pathway is evidenced involving the formation of supported defective CoS2 species transformed around 200−250 °C into supported Co9S8 species. Upon cooling down under sulfiding atmosphere, a reverse transition towards CoS2 was observed by XAS multivariate analysis and supported by Raman analysis. This reverse transition is sensitive to the Co9S8 crystallite size: the smaller the Co9S8 nanoparticles the higher the amount of formed CoS2 after cooling. The knowledge gained on the sulfidation of monometallic cobalt-based catalysts has been used in order to isolate the X-ray absorption spectrum of the CoMoS active species formed during the sulfidation of a calcined cobalt-promoted HDS catalyst. The evolution of the concentration of the CoMoS species upon temperature increase is determined for the first time, together with those of the pristine oxidic CoMo catalyst, CoS2 and Co9S8 supported species. Although the conversion of Co9S8 into CoS2 is still observed for the HDS catalyst when temperature is decreased under H2S/H2 atmosphere, no change of the CoMoS percentage is evidenced.
Journal Title: Catalysis Today
Year Published: 2020
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!