Photo from wikipedia
Summary The neuronal protein complexin contains multiple domains that exert clamping and facilitatory functions to tune spontaneous and action potential-triggered synaptic release. We address the clamping mechanism and show that… Click to show full abstract
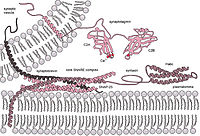
Summary The neuronal protein complexin contains multiple domains that exert clamping and facilitatory functions to tune spontaneous and action potential-triggered synaptic release. We address the clamping mechanism and show that the accessory helix of complexin arrests assembly of the soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) complex that forms the core machinery of intracellular membrane fusion. In a reconstituted fusion assay, site-and stage-specific photo-cross-linking reveals that, prior to fusion, the complexin accessory helix laterally binds the membrane-proximal C-terminal ends of SNAP25 and VAMP2. Corresponding complexin interface mutants selectively increase spontaneous release of neuro-transmitters in living neurons, implying that the accessory helix suppresses final zippering/assembly of the SNARE four-helix bundle by restraining VAMP2 and SNAP25.
Journal Title: Cell reports
Year Published: 2020
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!