Photo from wikipedia
Abstract As a consequence of the widespread use of polyethylene terephthalate (PET), huge amounts of PET waste are generated annually, and thus a critical point of waste management strategy is… Click to show full abstract
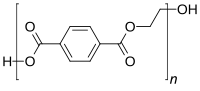
Abstract As a consequence of the widespread use of polyethylene terephthalate (PET), huge amounts of PET waste are generated annually, and thus a critical point of waste management strategy is to reduce these amounts. This study presents the chemical recycling of PET waste using sub- and supercritical water (SubCW and SCW). Two types of PET waste were chosen to study hydrolytic depolymerization: colorless and colored bottles. The experiments were carried out in a batch reactor at temperatures from 250 to 400 °C, with reaction period of 1–30 min. During the hydrolysis of PET waste, primary and secondary products were formed. The highest yield of terephthalic acid (TPA) was identified at 300 °C and a reaction period of 30 min; 90.0 ± 0.4% yield was observed from colorless PET waste and 85.0 ± 0.2% from green PET waste. The purity of final TPA obtained from PET waste was near 100%. The formation of secondary products such as benzoic acid, 1,4-dioxane, acetaldehyde, isophthalic acid (IPA) and carbon dioxide (CO2) were detected.
Journal Title: Chemical Engineering Science
Year Published: 2021
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!