Photo from wikipedia
Antibiotics represent a novel type of environment pollutants which modify chlorophyll content in plants. Spectroscopic methods were employed to investigate the effect of tetracycline on chlorophyll degradation. Changes in absorbance… Click to show full abstract
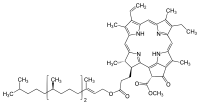
Antibiotics represent a novel type of environment pollutants which modify chlorophyll content in plants. Spectroscopic methods were employed to investigate the effect of tetracycline on chlorophyll degradation. Changes in absorbance and fluorescence demonstrated that tetracycline reaction with chlorophyll results in the formation of pheophytin, which was confirmed by new bands typical of pheophytin which appeared in the absorbance spectrum. The rate of pheophytin formation depended on ratio tetracycline to chlorophyll concentration in solution. In solutions with chlorophyll concentration of C = 1 × 10-5 M and tetracycline concentrations of C = 1 × 10-3 M and C = 1 × 10-2 M, pheophytin was formed after 28 h and 25 min, respectively. The obtained lifetime for pheophytin formed during chlorophyll reaction - with tetracycline hydrochloride was τ = 5.71 ± 0.02 ns and its value coincides, within the error limits, with the value obtained for pure pheophytin purchased from ChromaDex. The experiment demonstrated two mechanisms of chlorophyll degradation to pheophytin by tetracycline hydrochloride, i.e. 1) loss of Mg2+ ions from the chlorophyll molecule as a result of the presence of H+ ions in solution (i.e. as a result of medium acidification), and 2) removal of Mg2+ ions directly from chlorophyll by tetracycline which binds Mg2+ ions from the chlorophyll. We demonstrated that magnesium occurring in low concentrations attached to a tetracycline molecule in the BCD ring, and that the second ion of Mg2+ may attach to the A ring of tetracycline at higher Mg2+ concentrations. Two fluorescence bands appeared which indicated such magnesium attachments indeed occurred.
Journal Title: Chemosphere
Year Published: 2019
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!