Photo from wikipedia
ABSTRACT Viral and fungal dermatological manifestations are often the first and only sign of HIV/AIDS. They are cosmetically disfiguring and threaten the quality of life, but can be treated by… Click to show full abstract
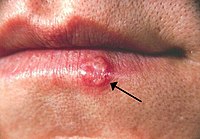
ABSTRACT Viral and fungal dermatological manifestations are often the first and only sign of HIV/AIDS. They are cosmetically disfiguring and threaten the quality of life, but can be treated by acyclovir and ketoconazole, correspondingly. This study attempted the formulation of stable, double fixed‐dose acyclovir and ketoconazole novel transmucosal dosage forms, which are able to provide an efficient flux for both compounds. A cream, gel and lip balm formulation containing both drugs were formulated that can be applied to mucosal tissue. Compatibility experiments between acyclovir and ketoconazole were conducted. A six month stability study was performed on the formulations which included visual appearance; mass variation; assay of the drugs; pH; viscosity; zeta potential; and particle size distribution. Flow‐through diffusion tests, utilizing vaginal porcine mucosal specimens, were conducted to determine in vitro permeation. No physicochemical interactions exist between the actives. Stability testing revealed that the formulations could be considered stable and may easily be stored at 25 °C/60% RH. The following rank order could be established for the average cumulative acyclovir amount that permeated the mucosa: Acitop® ≥ gel > cream > lip balm; and for the average cumulative ketoconazole amount: lip balm >>> Ketazol® > gel > cream. Both drugs depicted release rates that obeyed the Higuchi model, affirming release from a matrix system. Stable transmucosal dosage forms containing a double fixed‐dose of acyclovir and ketoconazole; and targeting mucosal tissue could thus be prepared. These formulations were able to provide an efficient flux for both compounds. Graphical abstract Figure. No Caption available.
Journal Title: European Journal of Pharmaceutical Sciences
Year Published: 2018
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!