Photo from wikipedia
Abstract There is an increasing demand for electric vehicle batteries with higher energy densities that can be manufactured with low cost utilizing environmentally friendly processes. Lithium nickel cobalt manganese oxides… Click to show full abstract
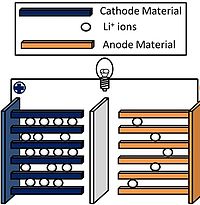
Abstract There is an increasing demand for electric vehicle batteries with higher energy densities that can be manufactured with low cost utilizing environmentally friendly processes. Lithium nickel cobalt manganese oxides (NCM), such as LiNi0.5Co0.2Mn0.3O2 (NCM523), are commonly used in commercial lithium ion batteries for electric vehicle applications. These cathodes are typically fabricated using environmentally hazardous, energy intensive, and time-consuming solvent-based slurry casting processes. Polymeric binders, also required for cathode preparation, add weight and can undergo parasitic reactions during battery cycling. Here we report a facile, solvent-free, binder-free, and rapid cold-press electrode fabrication process to prepare high mass loading NCM cathodes within minutes. This process is enabled by the use of holey graphene, a unique lightweight, compressible carbon nanomaterial that serves as both a binder and an electrically conductive matrix. NCM cathodes fabricated by this method are stable and exhibit good active material utilization. A significant advantage for the dry-press process is that the electrode mass loadings can be readily increased simply by adding more composite powder to the compression molding die, resulting in improved areal capacities that are attractive for applications requiring high energy per unit footprint.
Journal Title: Electrochimica Acta
Year Published: 2020
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!