Photo from wikipedia
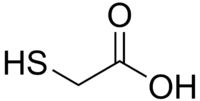
Abstract This study aims to investigate the thermal behaviours of a specific biomass and a polymer sample through co-pyrolytic degradation. Therefore, a non-edible food processing waste (cherry seed: CS) and… Click to show full abstract
Abstract This study aims to investigate the thermal behaviours of a specific biomass and a polymer sample through co-pyrolytic degradation. Therefore, a non-edible food processing waste (cherry seed: CS) and a typical plastic waste (polyvinyl chloride (PVC)) were selected and their interaction was studied using a combined TGA/MS/FT-IR system. By the help of TGA data, the non-isothermal kinetic analysis was performed using four different kinetic methods [Friedman, Flynn-Wall-Ozawa (FWO), Vyazovkin and distributed activation energy (DAEM)]. The kinetic results showed that the activation energy values were in agreement over the conversion degree values between 0.1 and 0.9. For determination of the synergistic or inhibitive interactions, theoretical and experimental thermogravimetry values were also compared. Moreover, in situ determination of the main pyrolytic and co-pyrolytic degradation compounds such as methyl, water, methoxy, hydrogen chloride, carbon dioxide and benzene with respect to temperature and time was studied for the first time. By this way, primary and secondary reactions related to methylation, dehydration, aromatization, fragmentation, dehydrochlorination were interpreted. Consequently, PVC was found to change pyrolytic degradation behaviour of lignocellulosic biomass by changing reactivity, activation energy and reaction mechanisms.