Photo from wikipedia
A series of nanomaterials have been demonstrated to be powerful for direct degradation of diethyl paraoxon (EP) to diethyl phosphate and 4-nitrophenol in aqueous solution. However, comparison of catalytic activity… Click to show full abstract
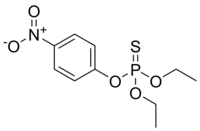
A series of nanomaterials have been demonstrated to be powerful for direct degradation of diethyl paraoxon (EP) to diethyl phosphate and 4-nitrophenol in aqueous solution. However, comparison of catalytic activity of different nanomaterials toward EP is rarely explored. In the present study, four different morphological nanoceria (cubes, rods, polyhedral, and spheres) were synthesized, characterized, and evaluated as a catalyst for the degradation of EP in comparison to other commercially available nanomaterials. Among the tested nanoceria, the cerium dioxide (CeO2) nanopolyhedra possess the best catalytic activity toward the hydrolysis of EP owing to their abundant oxygen vacancy sites, optimal ratio of Ce(III) to Ce(IV), and specific exposed facets. Under the conditions of 0.2 M NH3/NH4Cl buffer and 25 °C, the CeO2 nanopolyhedra catalyzed the reduction of EP to 4-nitrophenol with a >99% conversion at pH 8.0 for 50 h, at pH 10.0 for 12 h, and at pH 12.0 for 2.5 h. The catalytic degradation of nearly 100% EP in NH3/NH4Cl buffer (pH 10.0) at 25 °C is in the decreasing order of CeO2 nanopolyhedra > CeO2 nanorods > ZnO nanospheres (NSs) > CeO2 nanocubes > TiO2 NSs > CeO2 NSs > Fe3O4 NSs ~ Co3O4 NSs ~ control experiment. The mechanism for the degradation of EP was confirmed by monitoring catalytic kinetics of the CeO2 nanopolyhedra in the presence of EP, dimethyl paraoxon, 4-nitrophenyl phosphate, and parathion. The nanocomposites were simply fabricated by electrostatic self-assembly of the CeO2 nanopolyhedra and poly(diallyldimethylammonium chloride)-capped gold nanoparticles (PDDA-AuNPs). The resultant nanocomposites still efficiently catalyzed NaBH4-mediated reduction of 4-nitrophenol to 4-aminophenol with a normalized rate constant of 6.68 ± 0.72 s-1 g-1 and a chemoselectivity of >99%. In confirmation of the robustness and applicability of the as-prepared nanocomposites, they were further used to catalyze the degradation of EP to 4-amionphenol in river water and seawater.
Journal Title: Environmental research
Year Published: 2020
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!