Photo from wikipedia
Aripiprazole has been associated with impulse control symptoms (ICS). Recently, two drugs with similar pharmacological features have become available: cariprazine and brexpiprazole. All of them interact with the D3 receptor,… Click to show full abstract
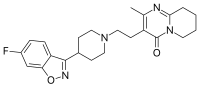
Aripiprazole has been associated with impulse control symptoms (ICS). Recently, two drugs with similar pharmacological features have become available: cariprazine and brexpiprazole. All of them interact with the D3 receptor, which plays a role in cerebral circuits involved in reward pathways. The objective of this study was to analyze whether a disproportionate number of cases of ICS are reported for cariprazine or brexpiprazole in EudraVigilance. A case/non-case study was conducted to assess the association between ICS and these antipsychotics, calculating reporting odds ratios (RORs) from their respective approval date to Nov 17, 2020. First, cases involving cariprazine or brexpiprazole were compared with those involving all other drugs. Second, to reduce the risk of confounding by indication, the RORs for cariprazine and brexpiprazole were compared with other antipsychotics. Besides, to evaluate a possible notoriety bias, a sensitivity analysis excluding aripiprazole was performed. Seven cases of ICS were reported for cariprazine and another seven for brexpiprazole. The ROR for cariprazine was 28.3 (95% confidence interval [CI], 13.4-59.8) and 33.4 (15.8-70.1) in the case of brexpiprazole. Nonetheless, this association disappeared for cariprazine when compared with other antipsychotics drugs. However, when excluding aripiprazole from the analysis, a safety signal emerged. Although our study is the first to suggest an association between cariprazine, brexpiprazole and ICS, these results should only be considered as exploratory in the context of safety signal detection. Further, well designed observational analytical studies will be needed to confirm these results.
Journal Title: European Neuropsychopharmacology
Year Published: 2021
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!