Photo from wikipedia
Abstract Ring-opening polymerization (ROP) of propylene oxide (PO) is achieved at 25 °C either in bulk or in solution, using N-heterocyclic carbenes (NHCs) and triisobutylaluminum (i-Bu3Al) as a bicomponent catalytic system.… Click to show full abstract
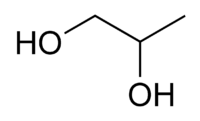
Abstract Ring-opening polymerization (ROP) of propylene oxide (PO) is achieved at 25 °C either in bulk or in solution, using N-heterocyclic carbenes (NHCs) and triisobutylaluminum (i-Bu3Al) as a bicomponent catalytic system. Transfer to monomer was not observed and poly(propylene oxide)s with predictable molar masses up to 60 000 g·mol−1 and low dispersities were obtained. In presence/absence of an alcohol as the initiator, the polymerization of PO follows anionic or zwitterionic ROP mechanisms, respectively. The addition of the Lewis acid strongly improves the efficiency of NHCs for the polymerization of substituted epoxides. It is established that i-Bu3Al is involved both in the formation of an initiating/propagating complex of moderate basicity/nucleophilicity and in the coordination of PO, enabling the activation of the monomer towards the complexed nucleophilic active species. Block copolyethers are also prepared by PPO chain extension experiments. All (co)polyethers were thoroughly characterized by 1H NMR spectroscopy, SEC and MALDI-TOF mass spectrometry as means to prove the control and benefit of this NHC approach for epoxides ROP.
Journal Title: European Polymer Journal
Year Published: 2020
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!