Photo from wikipedia
Abstract Trehalose and has been proven to be able to stabilize the native structure of biological system in extreme environments and widely used in cryopreservation. To understand the underlying mechanism… Click to show full abstract
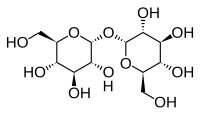
Abstract Trehalose and has been proven to be able to stabilize the native structure of biological system in extreme environments and widely used in cryopreservation. To understand the underlying mechanism of this stabilization effect, it is helpful to study the microscopic changes of membrane in the presence of varying amounts of trehalose upon rapid heating. In present work, molecular simulations were performed on the water/trehalose/POPC systems with different compositions. The results indicate that the stabilization of POPC membrane is greatly influenced by the content of trehalose as well as water. At a certain temperature, POPC membrane can be laterally expanded and transversely compressed upon addition of water or trehalose. The effective hydration level of bilayer is rising with the water or trehalose content increasing. The order degree of bilayer is almost insensitive to water content while it shows negative dependence on trehalose content, especially at lower temperature. On the other hand, as the temperature rises from 303 K to 373 K, the inclusion of trehalose significantly narrow the gap before and after heating for above parameters and this effect is more pronounced at higher trehalose content. With trehalose increasing from 0 to 256, the relative variation upon heating reduces by approximately 46.6% for area per lipid and 50.4% for bilayer thickness. That means other than improving the membrane structure at a certain temperature, trehalose also protects it from the injury due to structure change upon abrupt temperature variation. Further more, the essential mechanism is known as the slow mobility and strong hydrogen bonding (H-bonding) ability of trehalose. Compared with other cryoprotectants such as ethanol, trehalose dramatically slow down the movement of water, POPC and itself through H-bonds with longer lifetime between these molecules. Our findings could provide some insights in the design of protecting agents and processes.
Journal Title: Fluid Phase Equilibria
Year Published: 2018
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!