Photo from wikipedia
Abstract The effect of char during the heterogeneous reduction of N2O by CO has been studied using density functional theory (DFT). The armchair and zigzag configurations with six and seven… Click to show full abstract
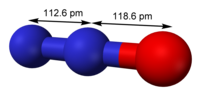
Abstract The effect of char during the heterogeneous reduction of N2O by CO has been studied using density functional theory (DFT). The armchair and zigzag configurations with six and seven aromatic ring clusters are selected as the carbonaceous surfaces. The calculation results show that heterogeneous reduction of N2O by CO on char surface undergoes two stages: N2O reduction and residual oxygen desorption. Char has a significant catalytic effect on the reduction of N2O by CO, which not only provides reactive sites for CO and N2O but also significantly reduces the activation energy of N2O reduction. On the one hand, the presence of CO significantly reduces the activation energy of N2 desorption on the char surface, and synergize with char to promote N2O reduction. On the other hand, it significantly reduces the activation energy of CO2 desorption and is beneficial to CO2 release. Char edge types have different catalytic effects on N2O heterogeneous reduction, and zigzag char is more advantageous in kinetics than armchair char. This study makes contributions to enrich the mechanisms of N2O reduction during combustion.
Journal Title: Fuel
Year Published: 2019
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!