Photo from wikipedia
Abstract Glucosamine is a monomer of chitin/chitosan, which is a renewable aquatic resource and the second most abundant biopolymer on the Earth. Moreover, methanesulfonic acid (MSA) is known as an… Click to show full abstract
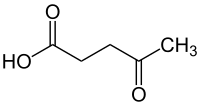
Abstract Glucosamine is a monomer of chitin/chitosan, which is a renewable aquatic resource and the second most abundant biopolymer on the Earth. Moreover, methanesulfonic acid (MSA) is known as an eco-friendly green catalyst. In this study, the MSA-catalyzed conversion of glucosamine to levulinic (LA) and formic (FA) acids was optimized using the Box-Behnken statistical approach. The optimal conditions for LA yield were 50 g/L glucosamine, 0.5 M MSA, 200 °C and 30 min, which yielded 49.9% LA and 50.8% FA. The LA yield increased linearly with increasing combined severity factor (CSF) until 3.5 and then, was maintained as the CSF value was further increased. The FA yield behaved similarly to LA. Both trends fitted well to a sigmoid regression model with a high regression value. These results highlight the potential of glucosamine for biofuel and chemicals production via an MSA-catalyzed conversion system.
Journal Title: Fuel Processing Technology
Year Published: 2018
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!