Photo from wikipedia
BACKGROUND Efficacious topical medications for rosacea are needed. FMX103 1.5% is a novel, topical minocycline foam that may have therapeutic benefits in treating rosacea, while minimizing systemic side effects due… Click to show full abstract
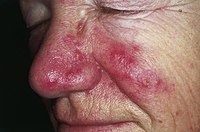
BACKGROUND Efficacious topical medications for rosacea are needed. FMX103 1.5% is a novel, topical minocycline foam that may have therapeutic benefits in treating rosacea, while minimizing systemic side effects due to its topical route of delivery. OBJECTIVE Determine the efficacy, safety, and tolerability of 12 weeks of treatment with FMX103 1.5% topical minocycline foam for papulopustular rosacea. METHODS Two 12-week, Phase 3, randomized, multicenter, double-blind, vehicle-controlled, 2-arm studies were performed in patients with moderate-to-severe papulopustular rosacea. RESULTS Subjects who received FMX103 1.5%, vs vehicle-treated controls, exhibited a significantly greater reduction in the number of inflammatory lesions (FX2016-11: -17.57 vs -15.65; P=.0031; FX2016-12: -18.54 vs -14.88; P<.0001) and higher rates of Investigator Global Assessment treatment success (FX2016-11: 52.1% vs 43.0%; P=.0273; FX2016-12: 49.1% vs 39.0%; P=.0077). No serious treatment-related treatment-emergent adverse events occurred. LIMITATIONS The generalizability of these data from a controlled clinical trial should be examined in a real-world setting. CONCLUSION FMX103 1.5% was efficacious for moderate-to-severe papulopustular rosacea, while maintaining a favorable safety profile.
Journal Title: Journal of the American Academy of Dermatology
Year Published: 2020
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!