Photo from wikipedia
The recent article by Zhao and colleagues [1] using a flexible lightwand to guide endotracheal tube (ETT) withdrawal and incision site determination during the percutaneous dilatational tracheotomy (PDT) in a… Click to show full abstract
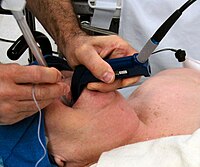
The recent article by Zhao and colleagues [1] using a flexible lightwand to guide endotracheal tube (ETT) withdrawal and incision site determination during the percutaneous dilatational tracheotomy (PDT) in a randomized controlled study was of great interest. They show that this method compared with the traditional technique can effectively obtain precise ETT repositioning and provide incision site confirmation with less intraand postoperative complications. Other than the limitations described in the discussion, however, we noted several issues of this study that need to be clarified and discussed. First, the authors did not clearly describe the manufacturer's details of the flexible lightwand used in this study. It is unclear whether this flexible lightwand is a registered medical device and how interested authors can purchase this device. Such information is useful for others who would like to try the PDT with this device. Moreover, the readers were not provided with details of the flexible lightwand, especially its outer diameter. According to Fig. 2 provided by authors, the outer diameter of the flexible lightwand should be N4.0 mm. We are concerned that when such thick flexible lightwand is inserted into the ETT to reposition the ETT and confirm the incision site, ventilation resistance will be significantly increased with adverse outcomes, especially for critical ill patients requiring a PDT [2]. Second, the authors stated that thedistance between ETT tip and cuff root was 3 finger widths (about 4.8 cm). However, the size and manufacturer of ETT used in this study were not provided. It must be emphasized that the distance between ETT tip and cuff root is significantly different among ETTs of various sizes from same manufacturer and among same size ETTs from various manufacturers [3]. Thus, the findings of this study may be not meaningfully extrapolated to other ETTs besides that used in this study. Third, it is generally recommended that during intubation, the ETT tip is passed through the glottis, stopping 2 cm after the cuff completely passes the glottis; alternatively, when the external tube markings of 22 to 24 cm are at the upper incisors, the tube tip is at the midtrachea [4]. In this study, for Group W patients, the
Journal Title: Journal of critical care
Year Published: 2017
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!