Photo from wikipedia
Abstract We have observed the solution structure of the supercooled sodium acetate aqueous solution, especially for the existence of clusters and their crystallization process, by means of Scanning electron microscopy… Click to show full abstract
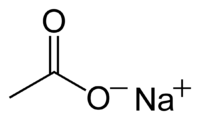
Abstract We have observed the solution structure of the supercooled sodium acetate aqueous solution, especially for the existence of clusters and their crystallization process, by means of Scanning electron microscopy (SEM) with the freeze replica method. Microscopic internal structure of sodium acetate trihydrate crystals mainly constitutes the aggregates of 100–200 nm in diameter, which consists of the clusters of 10–20 nm in diameter. In the case of a supercooled aqueous solution of 293 K, two types of aspect in the vitrified aqueous solution mainly exist: one is the clusters of 10–20 nm in diameter; the other is the smooth zone without any structure. At 263 K, the relationship among clusters of 10–20 nm and their aggregates of 100–200 nm was clearly observed. The aggregates construct the three-dimensional loose networks, which are not fully packed, different from the crystal.
Journal Title: Journal of Crystal Growth
Year Published: 2017
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!