Photo from wikipedia
Exenatide extended release is a once-weekly injectable medication used for the treatment of type II diabetes mellitus. The long-acting formulation consists of the original twice-daily formulation encapsulated in 0.06-mm-diameter microspheres.1… Click to show full abstract
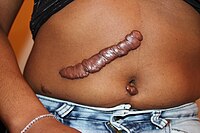
Exenatide extended release is a once-weekly injectable medication used for the treatment of type II diabetes mellitus. The long-acting formulation consists of the original twice-daily formulation encapsulated in 0.06-mm-diameter microspheres.1 The subcutaneously injected exenatide microspheres then diffuse the medication slowly over time, reaching therapeutic range by 2 weeks and steady state by 6 to 7 weeks.1 Pharmacologically, it acts as an incretin analogue that activates glucagonlike peptide 1 receptors causing a glucose-dependent insulin secretion.2 Exenatide reduces Hgb-A1C, fasting and postprandial glucose, and bodyweight.2 The most commonly reported adverse events include nausea, vomiting, diarrhea, and headache.2 Common adverse reactions at the injection site include pruritus, erythema, and subcutaneous nodules, which have reportedly resolved without intervention or limiting treatment.1, 3 These reactions are thought to be either an inflammatory foreign body reaction or an antiexenatide antibody response.1, 4 A handful of cases have also identified eosinophil-rich granulomatous panniculitis at the exenatide injection site, along with more persistent, nonresolving nodules.5, 6, 7, 8 For these rarer, persistent nodules, hospitalization and surgical intervention have been reported.8 We report a case of persistent exenatide extended release panniculitis nodules successfully treated with serial intralesional triamcinolone injections.
Journal Title: JAAD Case Reports
Year Published: 2018
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!