Photo from wikipedia
Abstract This work aimed to prepare activated carbons from coconut husk, a renewable source, and an agro-industrial waste, and different FeCl3 contents, resulting materials with magnetic properties. These materials had… Click to show full abstract
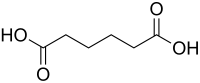
Abstract This work aimed to prepare activated carbons from coconut husk, a renewable source, and an agro-industrial waste, and different FeCl3 contents, resulting materials with magnetic properties. These materials had their performance tested in cyclohexene oxidation reactions in order to produce adipic acid, an important intermediate in the production of nylon 6.6 at mild conditions (catalyst 0.85 g, cyclohexene 0.90 g, H2O2 5.00 mL, acetonitrile 1.2 mL, time 24 h, temperature 75 °C and autogenous pressure). The catalysts were characterized by textural analysis, Boehm titration, XRD, SEM-EDS, TPD-He, LRS, Mossbauer spectroscopy and Magnetization curves. Adipic acid maximum yield (19 %) from cyclohexene was obtained with magnetic Fe3.7C catalyst (17.5 wt.% Fe) using H2O2 as oxidizing agent, no organic (acetic acid) or inorganic (HNO3) acid. The Fe/C catalysts prepared with activated carbon from a renewable source (coconut husk) produced adipic acid unlike other catalysts also prepared with FeCl3 in carbonaceous materials, such as RGO. Both higher acidity and concentration of oxidant α-FeOOH species were associated to cyclohexene oxidation to adipic acid. The products distribution followed three main pathways: 1,2-cyclohexanone rearrangement, allylic oxidation, and cyclohexene oxidation to adipic acid.
Journal Title: Journal of environmental chemical engineering
Year Published: 2020
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!