Photo from wikipedia
Abstract The galvanic displacement reaction between sacrificial PbO2 layers and Fe(II) ions has been carried out in mildly acid acetate solutions. The effect of experimental variables, like morphology of the… Click to show full abstract
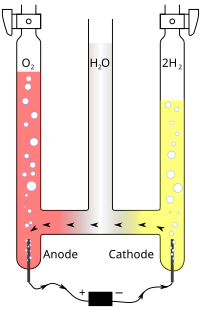
Abstract The galvanic displacement reaction between sacrificial PbO2 layers and Fe(II) ions has been carried out in mildly acid acetate solutions. The effect of experimental variables, like morphology of the PbO2 layers, solution pH, temperature and concentration of Fe(II) ions, has been investigated by combining electrochemical methods, SEM-EDS, XRD and XPS. The reaction between PbO2 and Fe(II) ions produced secondary dual layers, consisting of an inner amorphous FeOOH film and an outer crystalline γ-FeOOH deposit, respectively. This peculiar behavior was in contrast with analogous reactions involving other transition metal ions, which yielded only continuous amorphous secondary oxide layers. PbO2/FeOOH layers were tested as anodes for the oxygen evolution reaction in basic media.
Journal Title: Journal of Electroanalytical Chemistry
Year Published: 2020
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!