Photo from wikipedia
Abstract Polyglycolic acid (PGA), which is an important biodegradable polymer, can traditionally be synthesized through the ring opening polymerization of glycolide (with mostly using tin octanoate catalyst). Our previous studies… Click to show full abstract
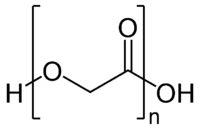
Abstract Polyglycolic acid (PGA), which is an important biodegradable polymer, can traditionally be synthesized through the ring opening polymerization of glycolide (with mostly using tin octanoate catalyst). Our previous studies revealed that PGA was alternatively synthesized with one-step cationic polymerization of formaldehyde from trioxane and carbonmonoxide (CO), sustainable C1 feedstocks obtainable from biomethanol or biogas. PGA and its copolymers can be mainly used for the biomedical applications due to their biocompatibility and biodegradability. In order to utilize PGA in other marketing materials such as packaging, PGA should be specifically engineered to improve its physical properties by a copolymerization strategy utilizing appropriate comonomers since PGA displays brown or beige color and is not soluble in most organic solvents due to its very high crystallinity. In this study; to improve on the physical properties of PGA, such as melting temperature and solubility, polymerizations of trioxane, CO and a minor amount of epoxides with long side chains were performed under the same reaction condition as PGA homopolymer synthesis (DCM solvent, at 800 psi, with triflic acid catalyst, reaction time of 72 h). The results have shown that optimum polymerizations were achieved at lower reaction temperatures than that of PGA homopolymer synthesis (110 °C versus 170 °C). The melting temperatures of all copolymers are lower, and the colors of the copolymers have become lighter than that of PGA homopolymer. The solubilities of obtained copolymers also increased by increasing side chain length of epoxides in the polymer backbone.
Journal Title: Journal of Saudi Chemical Society
Year Published: 2019
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!